Description
GSPR Checklist Excel Add-In – The Ultimate EU MDR 2017/745 Compliance Tool
Effortlessly Build Your GSPR Checklist for EU MDR Compliance
Are you struggling to compile a General Safety and Performance Requirements (GSPR) Checklist for EU MDR 2017/745 compliance? Our GSPR Checklist Excel Add-In and template is the ultimate tool to streamline the process, eliminate manual errors, and save you hours of work.
Designed for Regulatory Affairs professionals and Medical Device Manufacturers, this powerful GSPR checklist builder automates the creation, management, and export of a fully compliant GSPR checklist template—ensuring accuracy and efficiency at every step.
Key Features & Benefits
Automated GSPR Checklist Generation
-
Answer 19 key questions about your device, and the tool will automatically determine which GSPRs apply.
-
Auto-populate applicability statuses (Yes/No) and generate a rationale for non-applicable requirements.
Pre-Built & Customizable GSPR Checklist Template
-
Includes a structured GSPR checklist sheet with pre-built logic to guide compliance.
-
Fully editable and adaptable to meet your specific device requirements.
Document Integration & Auto-Population
-
Add and manage document references in a dedicated Documents Sheet.
-
Automatically link risk management, clinical evaluation, and technical documentation to corresponding GSPRs.
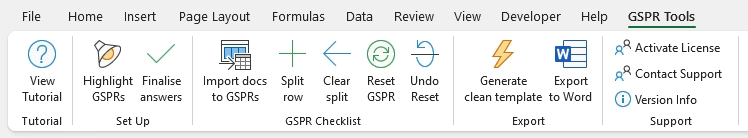
Smart Excel Ribbon Add-In for Easy Navigation
-
Adds a dedicated “GSPR Tools” tab to Excel for a seamless workflow.
-
Features buttons for highlighting applicable GSPRs, splitting rows for multiple document references, and resetting selections.
One-Click Export to Word
-
Convert your completed EU MDR GSPR Checklist into a professionally formatted Word document.
-
Ideal for regulatory submissions and audits.
Save Time & Ensure Compliance
-
Eliminates the hassle of manual GSPR checklist creation.
-
Reduces human error and improves consistency across compliance documentation.
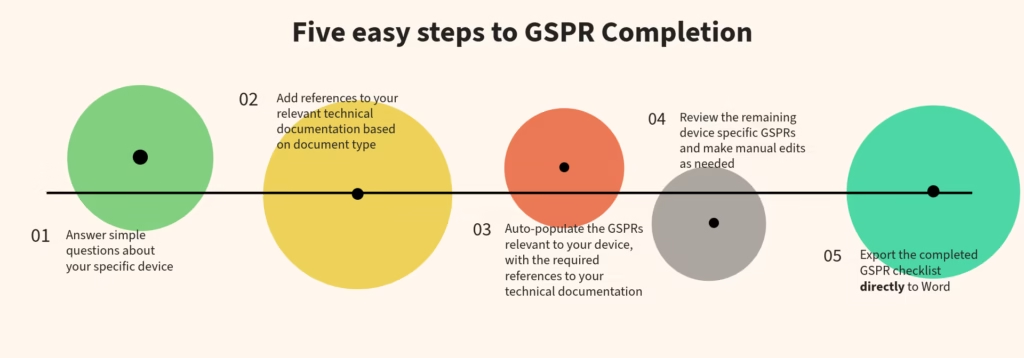
How It Works
- Setup Your Checklist: Answer the initial questionnaire to determine which GSPRs apply.
- Auto-Populate Your GSPRs: Import and link supporting documents to the checklist.
- Customize & Edit: Adjust applicability, add references, and manage documentation.
- Export & Submit: Convert your checklist into a Word document for submission or internal review.
Get Started Immediately
Instant Download – Get your copy immediately after purchase.
Comprehensive User Guide – Step-by-step instructions included.
Customer Support – Reach out anytime for assistance.


Reviews
There are no reviews yet.